Hi are you searching solutions of Haloalkanes and haloarenes for class 12? If yes then you have landed the right place.
Haloalkanes and Haloarenes are an important class of organic compounds containing halogen atoms. This chapter from NCERT Class 12 Chemistry covers their structure, properties, reactions, and uses. This tutorial provides a comprehensive guide to the chapter along with NCERT solutions for key questions.














Haloalkanes and Haloarenes
Haloalkanes and Haloarenes are halogen-containing organic compounds where one or more hydrogen atoms in a hydrocarbon are replaced by halogen atoms (F, Cl, Br, I).
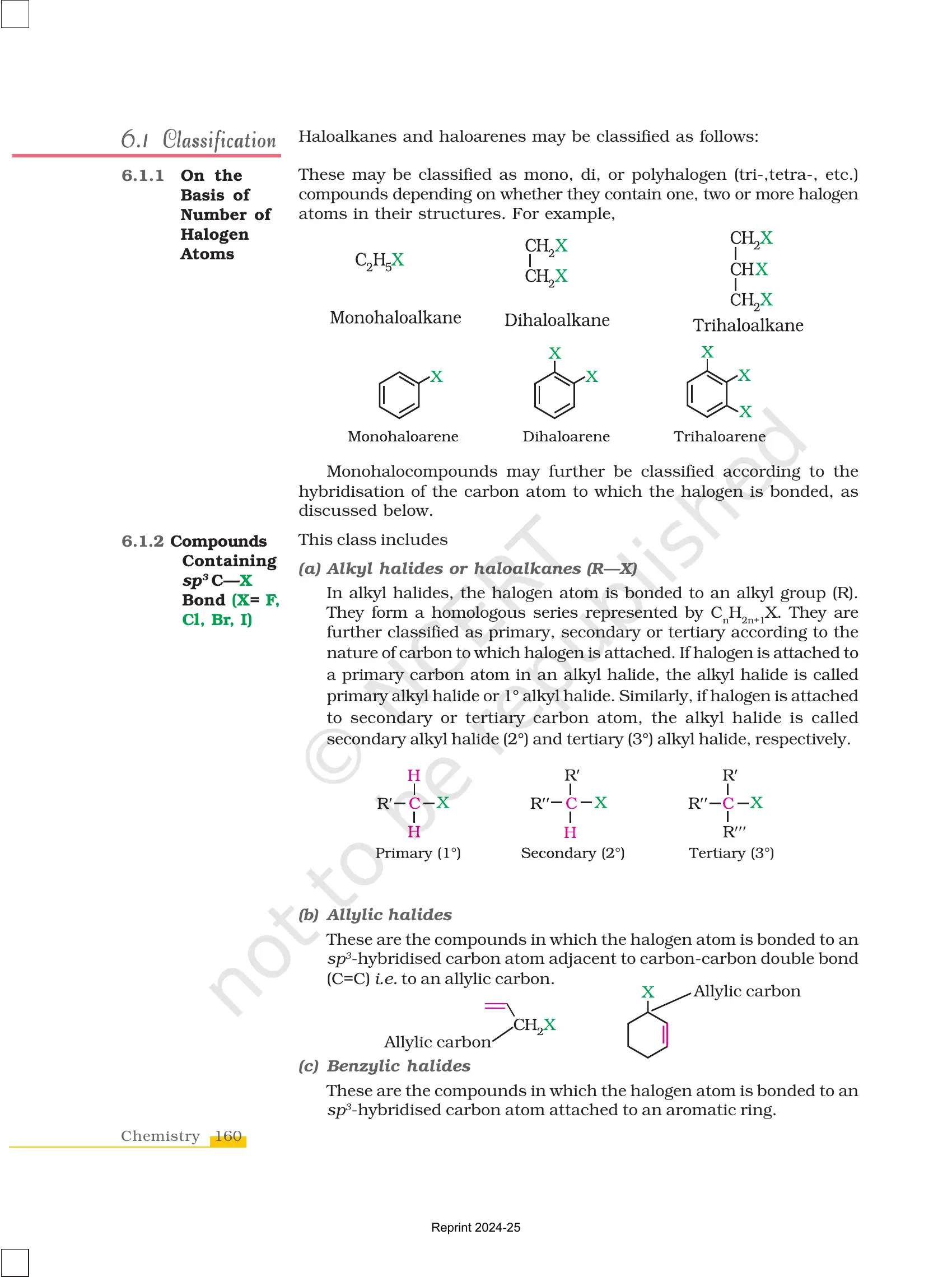
1. Haloalkanes (Alkyl Halides)
- Definition: Organic compounds where a halogen atom (X) is attached to an aliphatic carbon chain.
- General Formula: R-X (where R = alkyl group, X = halogen)
- Example:
- Methyl chloride (CH₃Cl)
- Ethyl bromide (C₂H₅Br)
Classification Based on Carbon Attachment:
- Primary (1°): Halogen attached to a carbon bonded to one other carbon. (Example: CH₃CH₂Cl)
- Secondary (2°): Halogen attached to a carbon bonded to two other carbons. (Example: CH₃CHClCH₃)
- Tertiary (3°): Halogen attached to a carbon bonded to three other carbons. (Example: (CH₃)₃CCl)
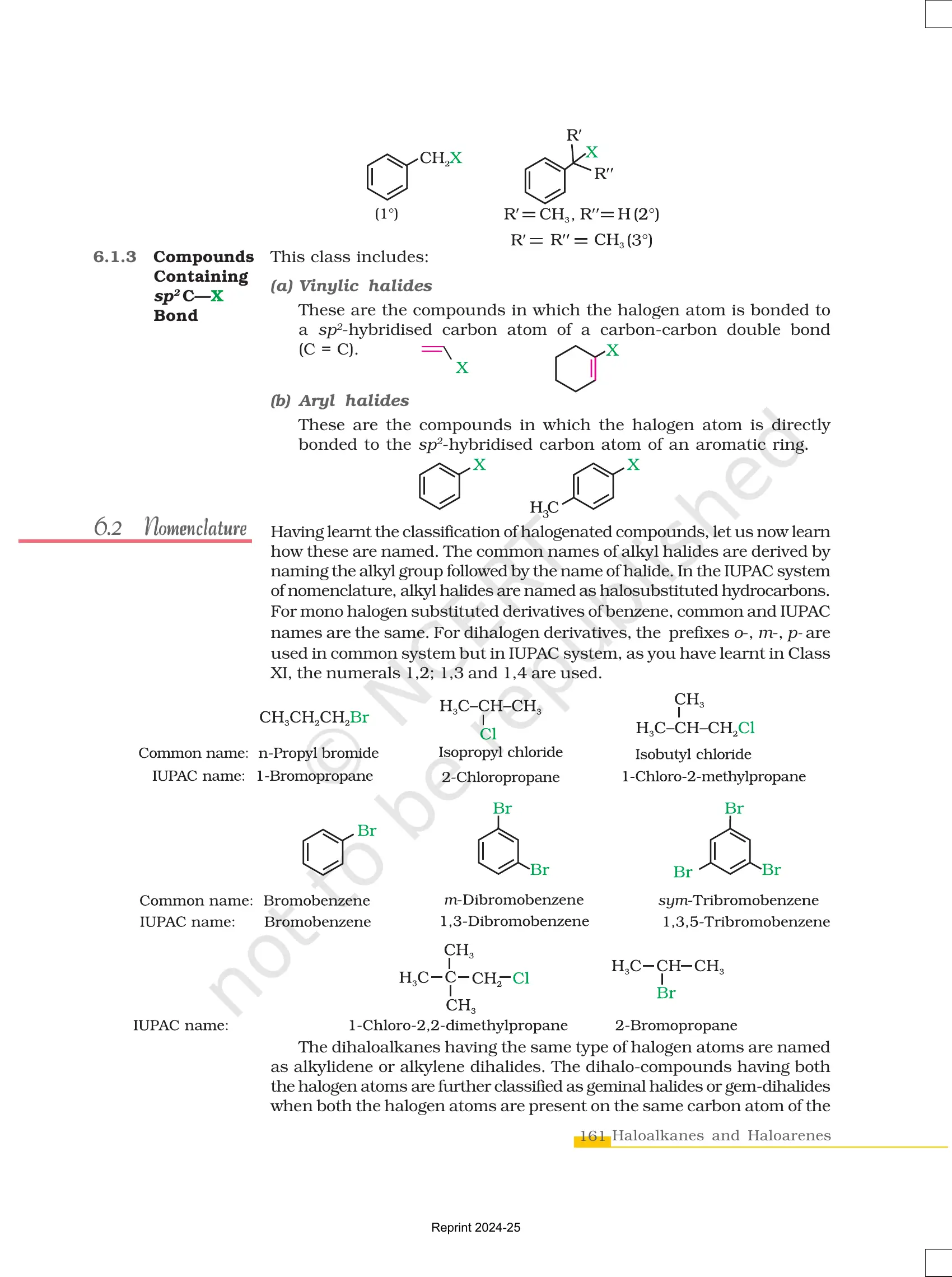
2. Haloarenes (Aryl Halides)
- Definition: Organic compounds where a halogen atom (X) is attached to an aromatic ring (benzene or derivatives).
- General Formula: Ar-X (where Ar = Aryl group, X = halogen)
- Example:
- Chlorobenzene (C₆H₅Cl)
- Bromobenzene (C₆H₅Br)
Key Differences from Haloalkanes:
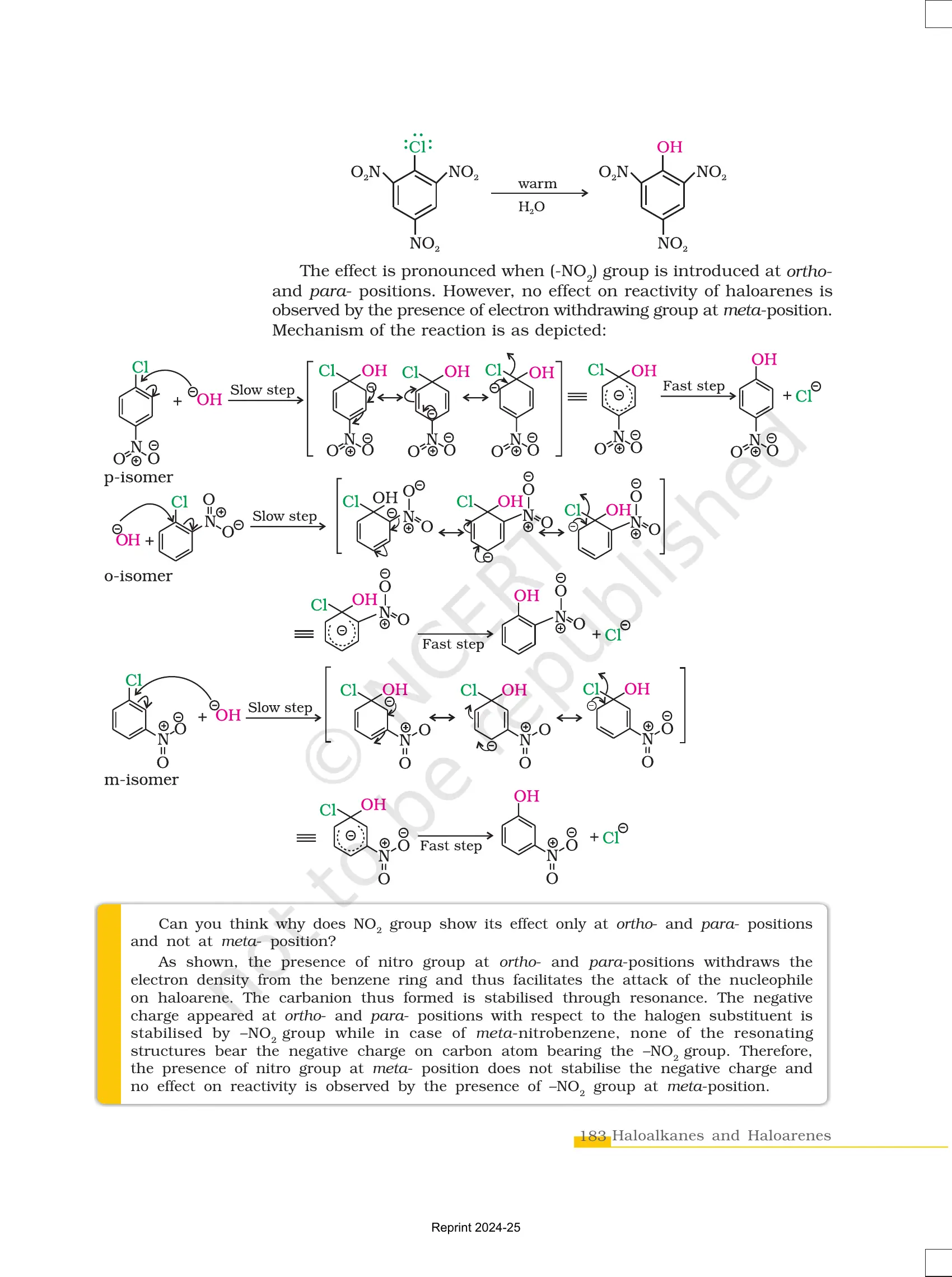
✔ Less reactive in nucleophilic substitution due to resonance.
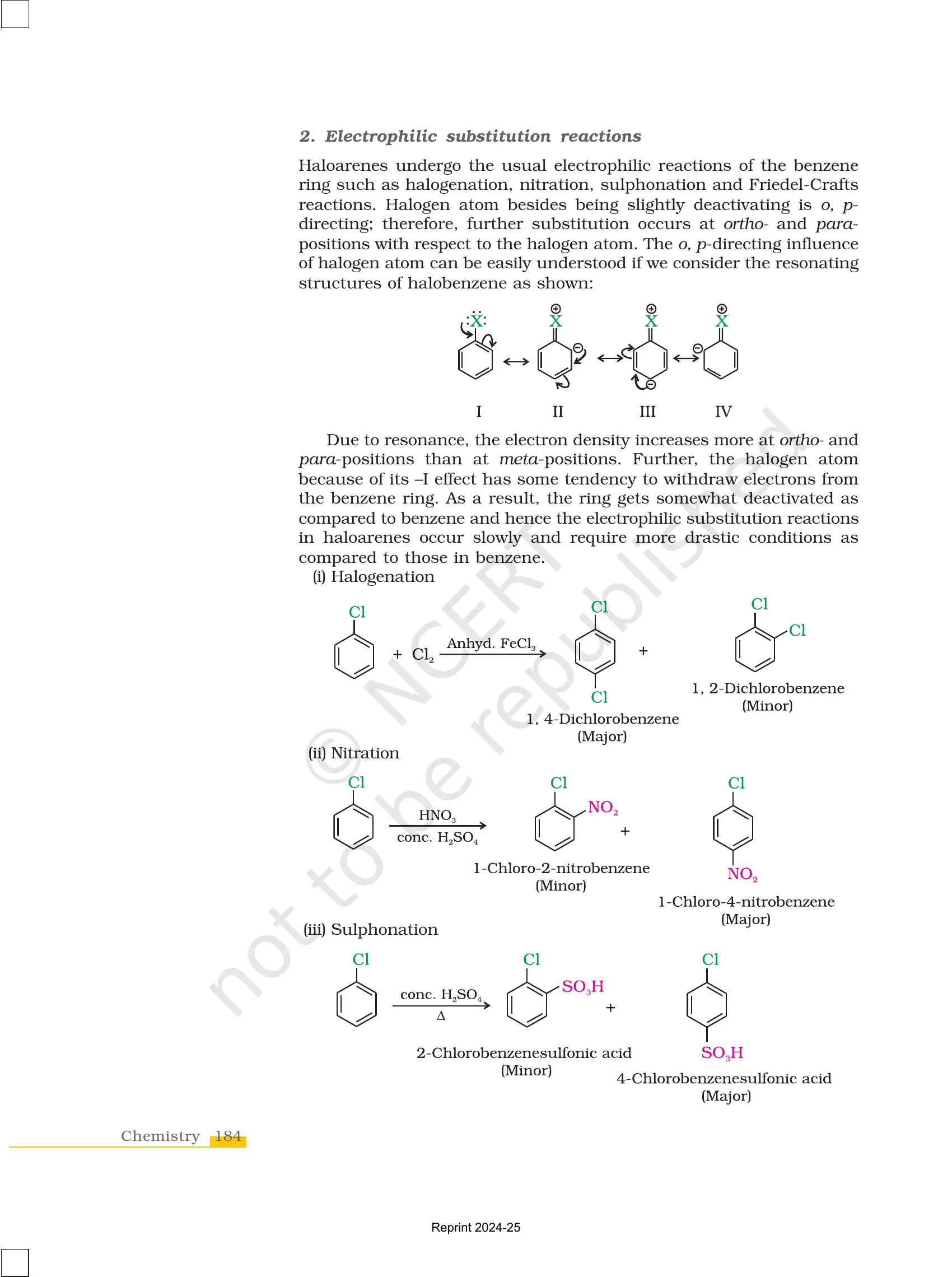
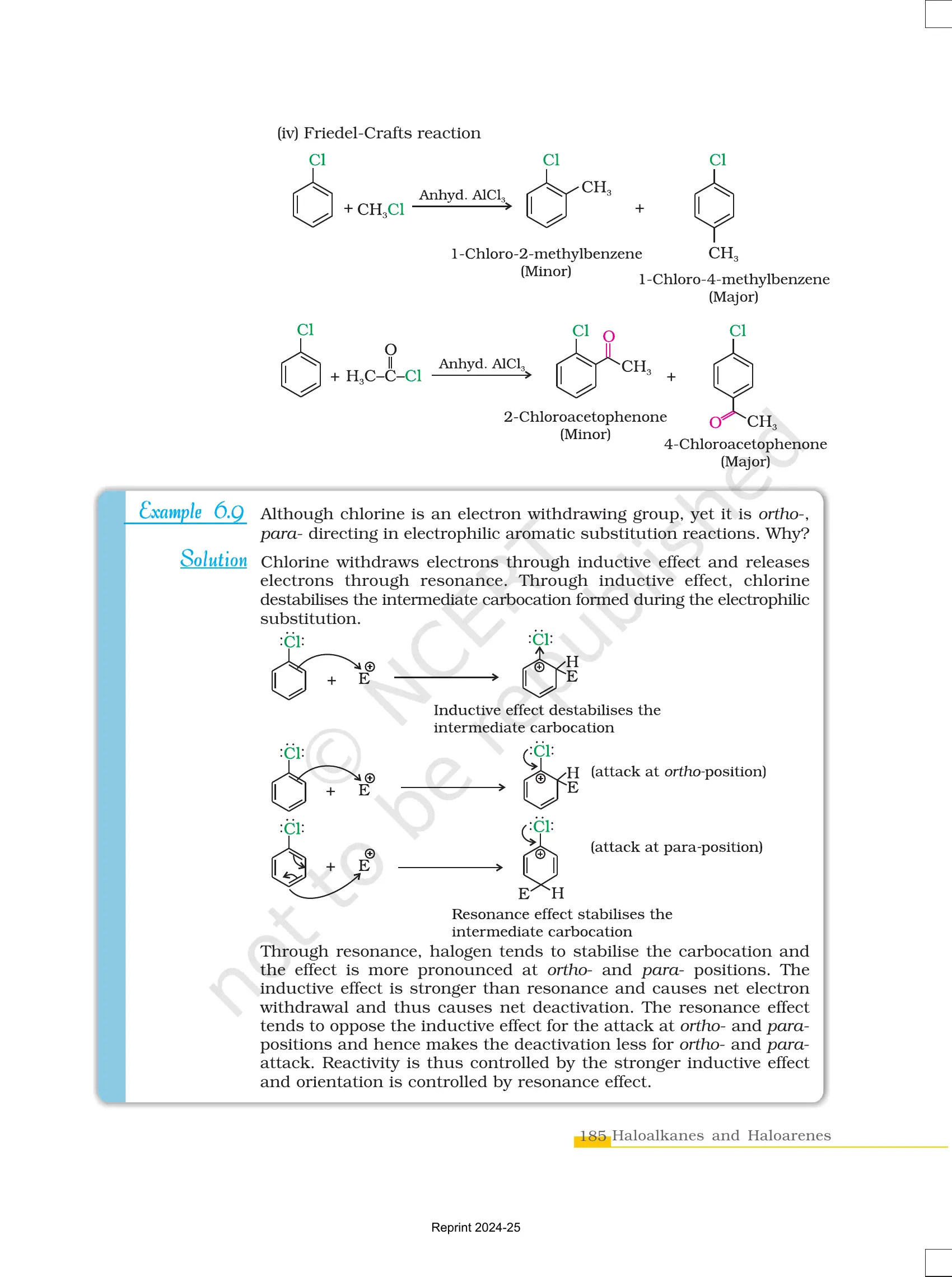
✔ Undergo electrophilic substitution reactions like nitration and sulphonation.
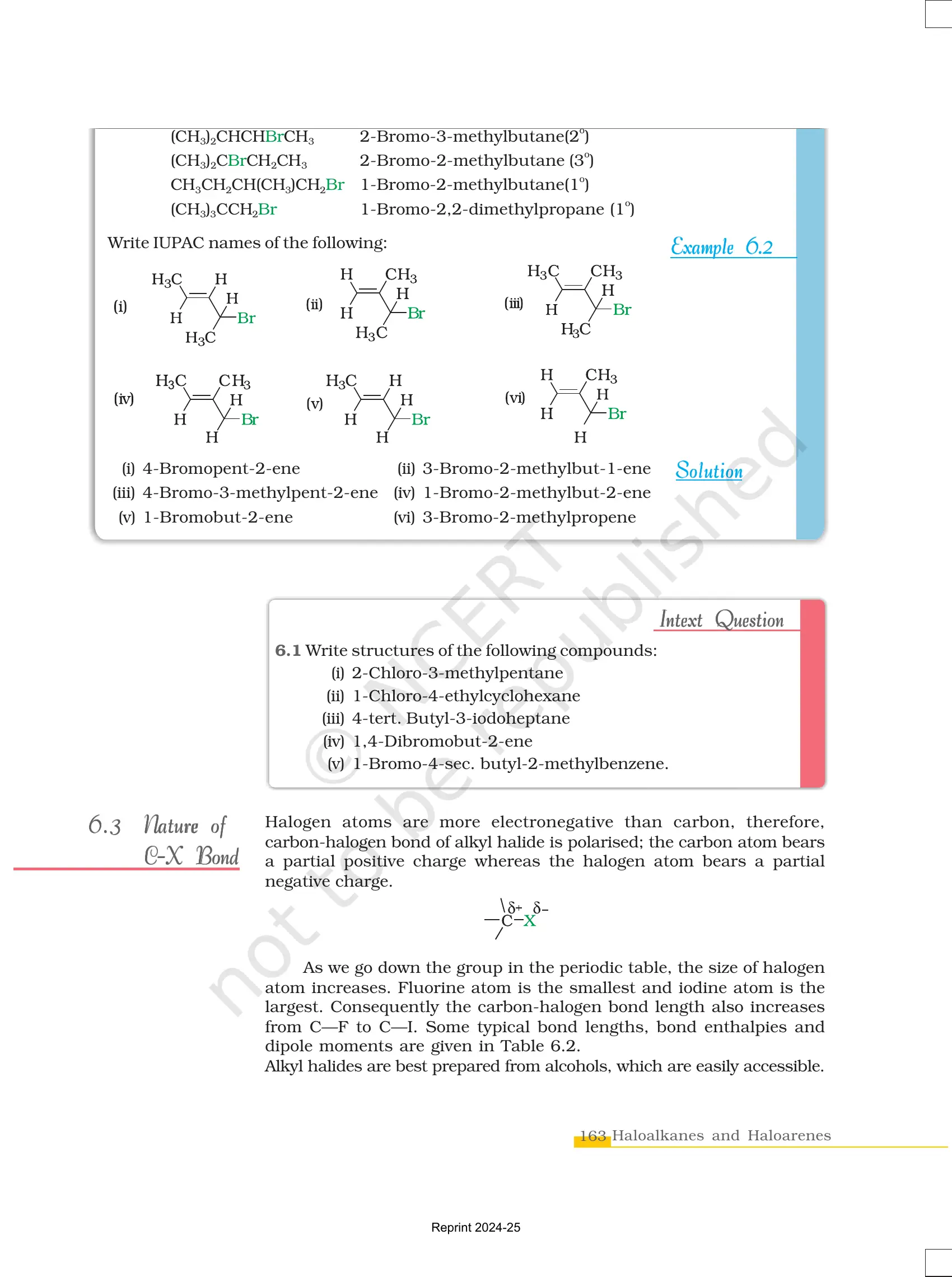
Classification and Nomenclature
Types of Halogen Compounds
- Haloalkanes (Alkyl Halides): Halogen atom attached to an aliphatic carbon chain.
Example: CH₃Cl (Methyl chloride) - Haloarenes (Aryl Halides): Halogen atom directly attached to an aromatic ring.
Example: C₆H₅Cl (Chlorobenzene)
IUPAC Naming Rules
- Identify the longest carbon chain containing the halogen.
- Number the chain so that the halogen gets the lowest possible number.
- Use prefixes like fluoro-, chloro-, bromo-, or iodo-.
Example: 2-Bromopropane (CH₃-CHBr-CH₃)
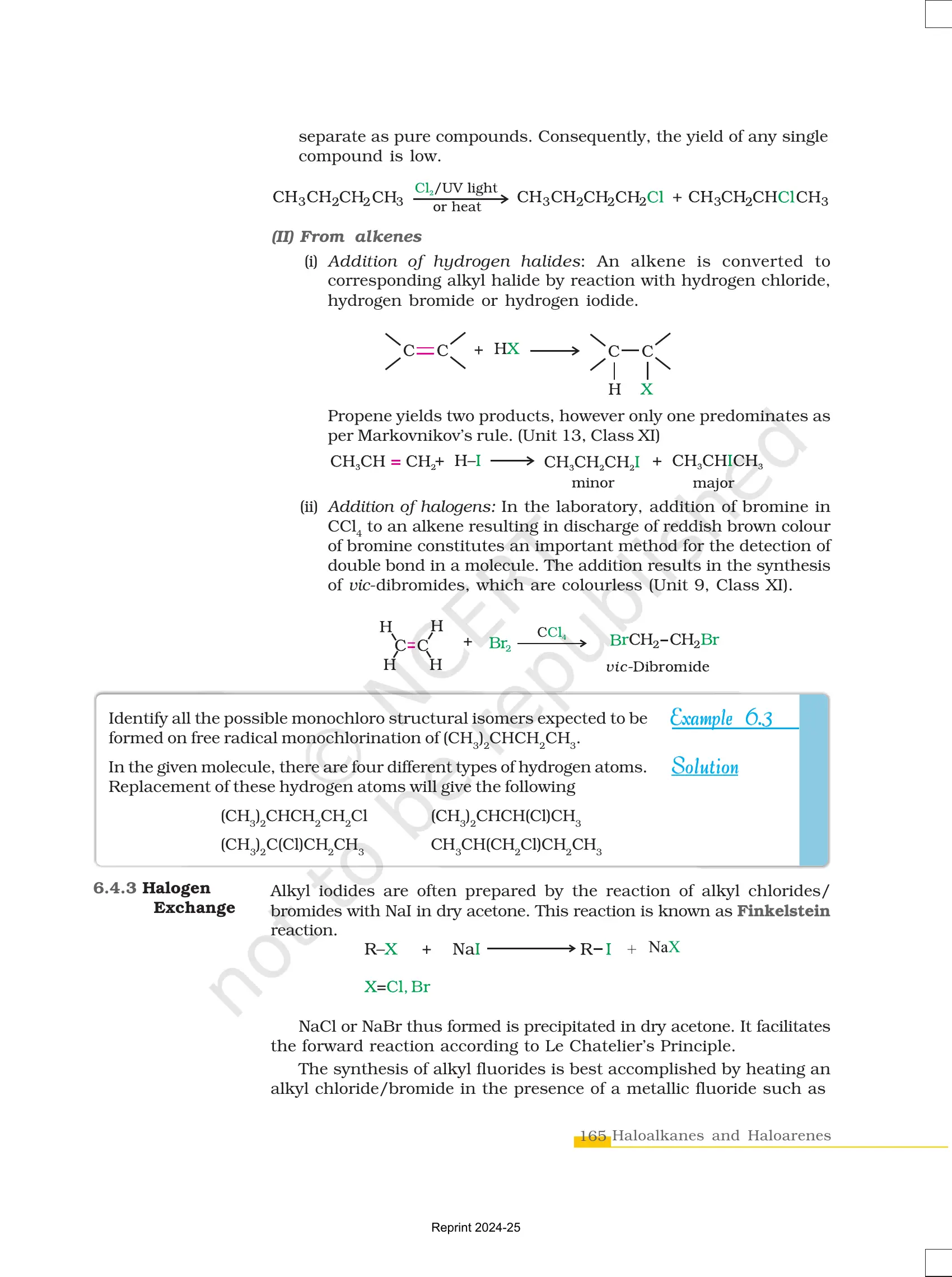
Nature of the C–X Bond
- The carbon-halogen (C–X) bond is polar due to the electronegativity difference between carbon and halogen.
- Bond strength decreases down the group: C-F > C-Cl > C-Br > C-I.
Conclusion
Haloalkanes and Haloarenes are crucial in organic chemistry and industrial applications. Understanding their reactions, properties, and environmental effects helps in solving NCERT problems effectively.
